How FDA Approval Costs for Generic Drugs Impact Healthcare Prices
 Dec, 12 2025
Dec, 12 2025
When you pick up a generic pill at the pharmacy, you probably assume it’s cheaper because it’s the same drug as the brand-name version. But what you don’t see is the hidden cost buried in the regulatory process - a cost that can delay your access, inflate prices, and even keep life-saving medications off the shelf for years. The FDA’s approval system for generic drugs isn’t just bureaucracy. It’s a financial engine that shapes who gets treated, when, and at what price.
The Real Cost of Getting a Generic Drug Approved
The FDA doesn’t approve generic drugs for free. Under the Generic Drug User Fee Amendments (GDUFA), companies pay fees just to get their application reviewed. For FY 2025, the total fee structure is $638.9 million. That includes $238,055 per manufacturing facility, $136,485 per product application, and additional application fees. For a single generic drug, the total cost to submit and get reviewed can hit $375,000 - and that’s just the start.
Compare that to brand-name drugs. A New Drug Application (NDA) under PDUFA costs $3.685 million. So yes, generics are cheaper to approve - but the gap isn’t as wide as you’d think. And for complex generics - things like inhalers, injectables, or topical creams - the real cost isn’t the fee. It’s the time, the failed attempts, and the uncertainty.
Why Complex Generics Take Years Longer
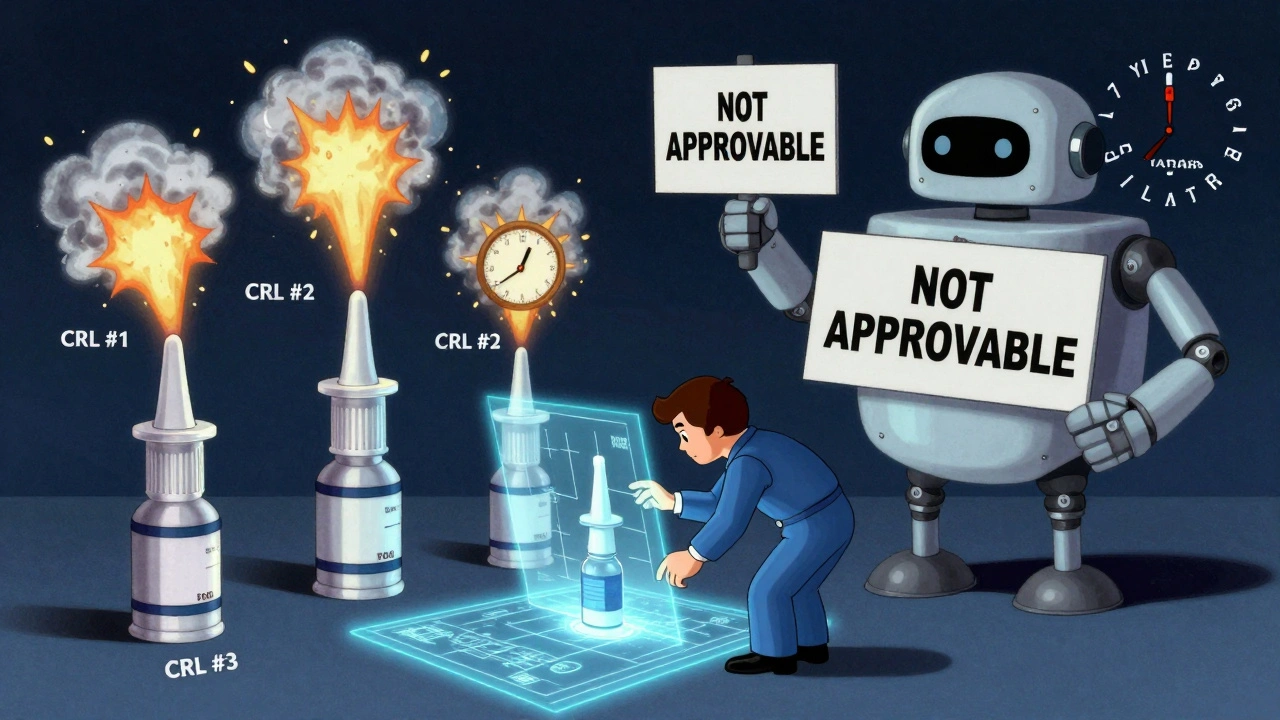
Not all generics are created equal. A simple tablet with one active ingredient? That’s straightforward. But a nasal spray with a precise particle size, or a cream with a specific viscosity? Those are complex generics. And since 2015, the FDA stopped giving manufacturers specific feedback on formulation issues. No more detailed letters saying, “Your particle size is 5% off” or “Your solvent ratio needs adjustment.” Instead, companies get vague Complete Response Letters (CRLs): “The application is not approvable.”
That one policy change turned development into a guessing game. One mid-sized generic company told RAPS they spent $8.7 million and three separate reformulations over seven years just to get a nasal spray approved. Each failure added $2-5 million and 8-12 months to the timeline. For a small company, that’s bankruptcy risk.
Result? Only 42% of complex generic applications get approved on the first try. For standard generics, it’s 65%. That gap isn’t about quality - it’s about clarity. And it’s costing patients.
How Delays Drive Up Drug Prices
When a generic can’t get approved, the brand-name drug stays on the market with no competition. That means patients pay full price - often 300% more. Between 2016 and 2020, generic versions of testosterone replacement therapy were delayed by 4.7 years. During that time, patients paid hundreds of dollars more per month.
On Reddit, patients are sharing stories: “I pay $1,200 a month for my glipizide because the generic got stuck in review.” “My apixaban prescription is $500. The generic would be $25. Why won’t they approve it?” These aren’t outliers. They’re symptoms of a broken system.
The data backs this up. In 2024, generics saved the U.S. healthcare system $467 billion - 90% of all prescriptions filled, but only 12% of total drug spending. Yet 83% of brand-name drugs still have no generic competition five years after patent expiry. Why? Because the FDA’s review process is too slow, too opaque, and too risky for manufacturers to invest in.
The Hidden Costs of Paperwork
It’s not just the fee or the reformulation. It’s the paperwork. A generic application today averages 150,000 to 200,000 pages. In 2013, under GDUFA I, it was 50,000. That’s a 35% increase in preparation costs, according to Deloitte. Companies now hire entire teams just to compile data, format documents, and meet evolving FDA standards.
And the timeline? GDUFA III promises 10 months for a standard review. But in 2023, the actual average was 11.2 months. For complex generics, it’s often 18-24 months - if they even get approved on the first try. Add in patent litigation delays (287 tentative approvals sat in limbo in 2024) and manufacturing issues (143 applications delayed), and you’ve got a system where the fastest path to market is often the longest.
Who’s Trying to Fix This?
In September 2025, Rep. Neal Dunn and Rep. Kevin Mullin introduced H.R. 1843 - the Increasing Transparency in Generic Drug Applications Act. It’s simple: force the FDA to give back detailed feedback on formulation issues. The Congressional Budget Office estimates this could cut approval times for complex generics by 18-24 months.
That’s not just faster access - it’s billions in savings. The CBO projects H.R. 1843 could generate $1.8-2.3 billion in annual savings once fully implemented, and up to $23 billion over five years. Over 70 lawmakers from both parties have signed on. The bill is set for committee markup in December 2025.
The FDA isn’t opposed to change - but they’re cautious. In July 2025, their Office of Generic Drugs warned that restoring detailed feedback without more staff could hurt review quality. They’re caught between speed and safety. Patients want speed. Regulators need safety. The solution? More resources - not less.

What’s Next for Generic Drug Approval?
The FDA is already moving toward GDUFA IV, the next funding cycle for 2028-2032. Industry stakeholders are pushing for a 3-5% annual fee increase to hire more reviewers. The agency also announced in September 2025 that it will streamline the review process for 15 high-priority complex generics by 2027.
Meanwhile, biosimilars - the next wave of lower-cost biologics - are getting more attention. The FDA is experimenting with faster pathways, and their success could set a precedent for how complex generics are handled too.
The message is clear: the current system works for simple generics. But for the drugs that treat chronic conditions, cancer, and autoimmune diseases - the ones patients rely on - the approval process is broken. It’s expensive, slow, and unpredictable. And patients are paying the price.
What Manufacturers and Patients Can Do
For generic manufacturers: Use Type II meetings with the FDA early. According to AAM, 78% of successful applicants did this. It cuts review time by over three months on average. Don’t wait until you’ve spent millions to find out you’re off-target.
For patients: Ask your pharmacist if your medication has a generic version pending. If it’s been over five years since the brand patent expired and you’re still paying high prices, you’re not alone. Contact your representative. Support bills like H.R. 1843. The system only changes when people demand it.
The cost of a generic drug isn’t just what’s on the label. It’s what’s buried in the paperwork, the delays, and the policy decisions made behind closed doors. Fixing it won’t be easy. But the savings - in money, in health, in lives - are too big to ignore.
How much does it cost to get a generic drug approved by the FDA?
The total cost to submit and get a generic drug approved by the FDA averages around $375,000. This includes facility fees ($238,055 per facility), product fees ($136,485 per application), and application fees. For complex generics, additional reformulation costs can push total spending to $8-10 million or more due to multiple failed submissions.
Why are some generic drugs delayed for years?
Complex generics - like inhalers, injectables, or topical creams - face delays because the FDA stopped providing specific feedback on formulation issues in 2015. Without clear guidance, manufacturers must guess what changes are needed, leading to multiple failed submissions, each costing millions and adding years to approval timelines. About 30% of complex applications require more than one review cycle.
Do generic drugs take longer to approve than brand-name drugs?
No - in fact, the FDA aims to approve generic applications faster than brand-name ones. The target for generic reviews is 10 months, while brand-name applications (NDAs) can take 12-18 months. But for complex generics, the actual review time is often 18-24 months due to reformulation delays and vague feedback from the FDA, making them slower to reach market than simpler generics.
What is GDUFA and how does it affect generic drug prices?
GDUFA, or the Generic Drug User Fee Amendments, is a program that lets the FDA collect fees from generic drug manufacturers to fund faster reviews. While it has improved approval timelines overall, the fees and complex requirements have raised the cost of entry. This discourages smaller companies from entering the market, reducing competition and keeping prices higher than they could be.
How much money do generic drugs save the U.S. healthcare system?
In 2024, generic drugs saved the U.S. healthcare system $467 billion. They made up 90% of all prescriptions filled but only 12% of total drug spending. Without generics, patients and insurers would pay hundreds of billions more each year for the same medications.
Is there legislation to fix delays in generic drug approval?
Yes. H.R. 1843, the Increasing Transparency in Generic Drug Applications Act, was introduced in September 2025. It would require the FDA to provide detailed feedback on formulation issues for complex generics. The Congressional Budget Office estimates this could reduce approval times by 18-24 months for these drugs and generate $1.8-2.3 billion in annual savings.

Keasha Trawick
December 14, 2025 AT 02:26The FDA’s GDUFA fees are basically a tax on affordability. $375K just to *apply*? That’s not regulation-it’s a gatekeeping scheme designed to keep small players out. And don’t get me started on the 150,000-page applications. It’s like they’re trying to drown innovation in bureaucracy. I’ve seen startups fold before they even hit submission because their VC said ‘no way’ to that kind of capital burn. The system isn’t broken-it’s *designed* this way.
Deborah Andrich
December 15, 2025 AT 16:14I’ve been on insulin for 12 years. My generic cost $15 a month until last year. Now it’s $87. No reason given. No warning. Just one day the pharmacy said ‘sorry, no generic available’. I called the manufacturer. They said the FDA stalled their application for 18 months. That’s not a delay. That’s a death sentence for people like me. And nobody in Congress seems to care until it’s their mom’s prescription.
nithin Kuntumadugu
December 16, 2025 AT 02:21lol FDA = Food and Drug *A**hole* 😂
they just wanna keep the pharma bros rich. 150k pages? bro i wrote my college thesis on napkin. they prob got a team of 12 interns just to alphabetize tabs in a word doc. #conspiracy #gdufa #bigpharma
John Fred
December 17, 2025 AT 11:45Big picture alert! 🚨
Here’s the win: H.R. 1843 could slash approval times by 2 years for complex generics. That’s not just $$$ saved-it’s lives. Think about it: a diabetic in Ohio gets their metformin generic 18 months sooner → $1,200/month → $25/month. That’s 48x cheaper. That’s not policy. That’s justice.
And yes-Type II meetings? DO THEM. 78% of successful applicants did. It’s not magic. It’s strategy. You don’t guess. You ask. And the FDA? They’ll tell you… if you make them.
Hamza Laassili
December 18, 2025 AT 23:54WHY ARE WE LETTING FOREIGN COMPANIES DO THIS?!?!!?!!
INDIA. CHINA. THEY MAKE THE DRUGS. WE MAKE THE PAPERWORK. WE PAY FOR THE FEES. WE WAIT. THEY GET RICH. THIS IS A TRAGEDY. AMERICA IS BEING ROBBED. WE NEED A WALL… BUT FOR DRUGS. NOT PEOPLE. DRUGS.
Also-FDA is probably just lazy. They got a 401k and a nap room. I bet they’re playing solitaire while my insulin sits in limbo.
Cole Newman
December 20, 2025 AT 07:17Did you know the FDA doesn’t even test the drugs? They just read the paperwork. So if you lie on page 87,421, they’ll approve it. That’s not oversight. That’s trust-fall therapy. And guess what? The companies know it. So they game the system. And we pay for it. In pain. In dollars. In lives.
Casey Mellish
December 20, 2025 AT 19:37As an Aussie who’s watched our PBS system negotiate generic prices directly with manufacturers, I can say this: the U.S. system is a carnival ride designed by lawyers. We don’t have user fees-we have bulk negotiation. Result? Our generics cost 1/5th of yours. And we still have safety. It’s not magic. It’s political will. You’ve got the data. You’ve got the bill. Now-where’s the outrage?
Emily Haworth
December 20, 2025 AT 21:27They’re not slow because they’re overworked. They’re slow because they’re scared. 😈
Remember the Vioxx scandal? FDA got roasted. Now they’re terrified of approving something that might kill someone-even if it’s 99.9% safe. So they demand 17 extra studies. They ask for a ‘bioequivalence curve’ measured in nanometers. They don’t care if the patient dies waiting. They care about their reputation.
And don’t even get me started on the ‘secret’ meetings between FDA and big pharma. I’ve seen the emails. It’s not a process. It’s a club.
Tom Zerkoff
December 22, 2025 AT 03:28It is imperative to underscore that the current regulatory paradigm, while ostensibly designed to ensure therapeutic equivalence, has inadvertently engendered a market structure characterized by oligopolistic inertia. The confluence of exorbitant user fees, non-transparent feedback mechanisms, and institutional risk aversion has precipitated a significant reduction in market entry dynamics among small and mid-sized manufacturers. Consequently, the anticipated cost-reduction benefits of generic pharmaceuticals are systematically undermined by structural barriers that disproportionately affect patients with chronic conditions requiring complex formulations. A systemic recalibration, predicated upon increased transparency and resource allocation, is not merely advisable-it is ethically obligatory.
Yatendra S
December 22, 2025 AT 15:28What if the real problem isn’t the FDA… but the idea that drugs should be ‘owned’ at all? 🤔
Life is a flow. Medicine is a flow. But we treat molecules like property. We patent the cure. We charge for the breath. The FDA is just the priest of this temple. The real sin? We let corporations hold the keys to survival. Maybe the answer isn’t more feedback letters… but no patents. No fees. No FDA. Just science. Shared.
…just saying.
Himmat Singh
December 23, 2025 AT 05:55While the author presents a compelling narrative, it is necessary to interrogate the underlying assumption that regulatory delay is inherently detrimental. The FDA’s caution is not inefficiency-it is epistemic responsibility. To rush approval is to gamble with human life. The $467 billion in savings, while statistically significant, does not account for the potential cost of adverse events stemming from substandard generics. One must ask: Is speed truly a virtue when the stakes are physiological integrity?